Videos tagged with “synthesis”
Found 31 videos • 252,526 total views
Alright, jOeCHEMists. Our Gen Chem foundations are strong. We handled Alkanes with ease. We've taken our Stereochemistry vitamins. Now begins what I consider the fun part of Organic Chemistry. In this series, we'll tackle Sn2, E2, Sn1, and E1, and we'll do so by taking each reaction pathway one at a time and exploring it in depth. Once we fully understand a pathway, we'll solidify our knowledge through LOTS of examples for that specific pathway. Then, once we have a good handle on all four, we'll put ourselves to the ultimate test and solve problems without knowing what kind of reaction they are. Our knowledge of Sn2, E2, Sn1, and E1 will allow us to decipher how a reaction will proceed based on the conditions present, and believe it or not, we'll have to lean on everything we've learned prior as well.
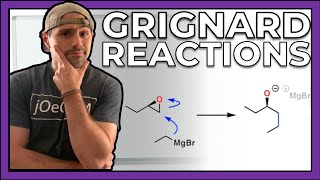
jOeCHEMists, I know I said it before, but NOW the real fun begins. Up until this point we've been filling our organic toolbox full of skills, and it's time we put our foot on the gas. It's time to learn reactions. It's time to learn mechanisms. It's time to learn how to design organic syntheses and solve synthesis problems. And we start this new chapter of our journey with alcohols. In this series, we're going to learn how to oxidize & reduce alcohols as well as how to perform Sn2 on them (even though alcohols aren't great leaving groups). But, most importantly, we'll learn the Grignard Reaction--marking a huge moment in our organic careers. The Grignard Reaction allows us to make carbon-carbon connections, and that will lead us straight into tackling our first synthesis problem. There's a lot of fun to be had in this section, gang. Let's get it.
Fresh off of our conquest of alcohols, we move straight into some relatives of the alcohol functional group: ethers and epoxides. We'll start things off with a recap and discussion regarding carbocation stability and shifts. Then, we'll move on to talk about reactions of ethers and how to use them as protecting groups. Finally, we'll round out the series by looking at attacking epoxides and how the regiochemistry of attacks changes based on acidic vs basic conditions.
jOeCHEMists, welcome to Organic Chemistry II. If you're here, that means you've taken on OChem I and came out the other side a stronger warrior than when you entered. Now, with one semester of chemistry in the books, we look forward to the second half of our carbon conquest, and we begin with Conjugation. In this series, we'll explore the stabilizing effect of lining up many carbons in a row that have p orbitals parallel to each other. After we understand conjugation, we'll see how that affects reaction we've done in the past, and then we'll round out the series with a brand new reaction--the Diels Alder reaction. So, gang: OChem II. Let's get after it.
Fresh off of our introduction to conjugation, we now understand the powerful stabilizing effect of having a network of consecutive atoms with unhybridized p orbitals parallel to one another. Well, in this series, we take it to the next level with the concept of aromaticity. Benzene is going to become our best friend in this series, and we'll see how, even though it is rock solid stable due to its aromaticity, to do chemical transformations with benzene and benzene derivatives. We'll learn a basic set of reactions, extra helper reactions, and we'll discuss how to control regiochemistry with various directing groups.
jOeCHEMists, now that we've explored more of the awesome chemistry we can do at the carbonyl position, we have to look no further than the next door neighbor for our next challenge: the alpha carbon. We'll start things off by exploring alpha deprotonation, why it works and how it enables us to generate a new class of nucleophiles that we were initially exposed to with keto-enol tautomerism at the end of OChem I. After learning how to make enolates and enols, we're going to tackle a group of reactions that allow us to make carbon-carbon bonds. There's so much cool chemistry in this series, and it's where I personally discovered that I wanted OChem in my future/life. I hope you feel the same. Leddoit.
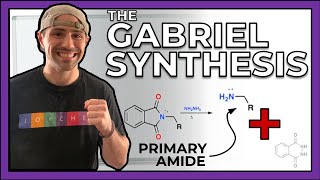
Up until this point, nitrogen was always that atom whose formal charge was a bit annoying to calculate. Yeah, we've seen nitrogen in a lot of acid-base reactions, and sure, nitrogen came back into the spotlight when we learned about amides. But now, nitrogen gets its five seconds of fame. In this series, gang, we do a deep dive on amines. We'll discuss their physical properties as well as how to produce them (in ways you already knew but also with new pathways). So buckle up: It's nitrogen time.
jOeCHEMists, let me hit you with a blast from the past: remember Fischer Projections? Well if you did or didn't, they're about to re-enter your world. In this series, we're going to cover carbohydrates. This is certainly an interesting section of Organic Chemistry, because while we will learn some new reactions & mechanisms, we won't spend the majority of our time doign that like we would in a typical section. We'll learn a lot of nomenclature, conventions, methods to draw/represent carbohydates, and a few reactions along the way. It'll be sweet, trust me (heh, get it? Yeah, I'm sure you did. Look, I had to do it, sorry.).
Gang, we've been hitting reactions and mechanisms REALLY hard. So we're going to take a little detour and tackle a topic in Organic Chemistry that is truly different than any other we've conquered thus far. In real life, after you've performed a reaction and have a product mixture, you want to verify that your reaction worked and see how much product you made. Well HNMR is the gold standard for determining the structure(s) present in a solution/sample.
Practice exam and review materials for Organic Chemistry I Exam 1
Practice exam and review materials for Organic Chemistry I Exam 2
Practice exam and review materials for Organic Chemistry I Exam 3
Comprehensive practice exam and review materials for Organic Chemistry I Final Exam
Hey, remember how much fun we had with the alpha carbon position? Those were some good times, huh? Well, let's run it back. In this section, we're going to be working with the alpha carbon again, but we'll be performing alpha deprotonation on esters, 1,3-dicarbonyls, and diesters. With this new class of enolate nucleophiles, we'll see the unique chemistry we can do, such as the Claisen Condensation, Decarboxylation, Malonic Ester Synthesis, and so much more.
If after the aromaticity + benzene series you found yourself craving more aromatic adventures, then I have some good news: you're in the right spot for round two. In this section, we massively deepen our knowledge regarding the chemistry we can do with benzene derivatives. We'll talk about ipso substitutions, EAS reactions with phenol, various rearrangements, and a whole lot more. We're even going to see a triple bond INSIDE of a benzene ring. It sounds whack. It sounds crazy. But, just like everything else we've learned together, we're going to master it.
Okay, gang, we've done A LOT of chemistry up to this point. We've seen many a reaction, countless mechanisms, and likely hundreds of cyclic structures. However, in all the rings we've seen, we haven't done a ton of chemistry with what are called heterocycles: rings with more than one type of atom in them. Well, that changes in this section. In this section, we'll be doing chemistry with cyclic structures that of course have carbon in them but also oxygen, nitrogen, and even sulfur. We'll even learn mechanistically how to make pyridine. The chemisty is cool and the rings are different--let's have ourselves a time.
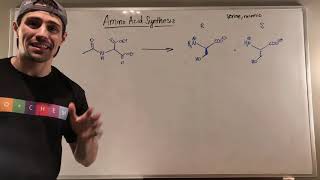
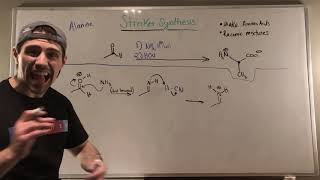
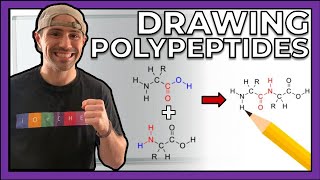
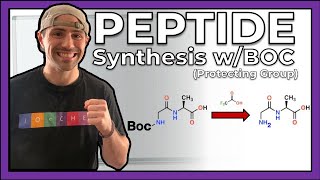
Learn about biologically relevant organic molecules.
Practice exam and review materials for Organic Chemistry II Exam 2
Comprehensive practice exam and review materials for Organic Chemistry II Final Exam